A computerized system can include hardware, software, its peripherals, interfaces, equipment, users, and operating procedures. Today, in the health products industry that encompasses pharmaceuticals, biologics, vaccines, biotechnology, natural health products, medical devices, cosmetics and allied industries, software and hardware components are used for the purposes of data processing, data storage, and process control.
AXSource Consulting is committed to understanding your unique situation and supporting a complete Computer System Validation (CSV) program, ensuring strict regulatory compliance.
According to US FDA, “Computer system validation is the process of providing a high degree of assurance through documented evidence that a computer system consistently meets its pre-determined or intended use or quality attributes such as accuracy, security, reliability, and functionality.”
We have performed Computer System Validation projects for custom, off-the-shelf (OTS) and highly configurable Enterprise Resource Planning (ERP) computer system applications such as:

We are fortunate to have AXSource Infotech that supports our design, development, and implementation efforts.
In addition to ERP, we have validated Laboratory Information Systems (LIMS/LIS), Electronic Data Capture (EDC) systems, and medical device software in “GxP” environments. All projects conform to recognized quality standards such as ISPE’s Good Automated Manufacturing Practices (GAMP 5 Guide: Compliant GxP Computerized Systems), US FDA 21 CFR Part 11 Regulations & FDA Software Validation Guidance, and EMA’s Annex 11 Regulations on Computerised Systems.
AXSource Consulting can assist in identifying the area of the regulated process or the medical devices that require validation. We can provide you with expert advice throughout the entire project lifecycle whether you are using an Agile Methodology or System Development Life Cycle (SDLC) approach.
Under FDA software validation requirements for medical devices, “any software used to automate any part of the device production process or any part of the quality system must be validated for its intended use,” as required by 21 CFR §820.70(i). This requirement applies to any software used to automate device design, testing, component acceptance, manufacturing, labeling, packaging, distribution, complaint handling, or to automate any other aspect of the quality system. We have ample experience performing medical device software validation for clients all over the world
Cost Savings
AXSource consultants prefer to support clients interactively to enable a smooth transition to client in-house IT and quality teams and most importantly to provide cost savings.
When is system or device validation required?
When is the right time to validate the system or device? To what extent is the validation necessary?
The extent of validation is commensurate to the risk posed by the system in terms of patient safety, the accuracy and security of the data involved, and/or the nature of the change. At AXSource, we evaluate the purpose and risks associated with each system prior to designing a validation strategy with our clients. Although not recommended by agencies, AXSource has also performed numerous retrospective validation of numerous existing computerized systems.
How does Software Validation apply to me?
Sample checklist of areas requiring software validation include:
1. Automated operation and/or control in “GxP” (Manufacturing, Laboratory, Clinical, Distribution) environments ✔
2. Software used as a medical device or as a component of a medical device; ✔
3. Controlling of health product (medical device, pharmaceutical, natural health product, biologics in humans or animals) manufacturing or control process, e.g., weighing, mixing, compounding, labeling, automated inspection systems, laboratory information system ✔
4. Used in traceability and inventory control of health product (raw materials, components, bulk, finished products, devices) ✔
5. Used in recording health product manufacturing and control batch history information (electronic Batch Record) including e-Signature ✔
6. Used in collecting & archiving clinical data to order to perform a medical assessment of risk & treatment ✔
7. Used in Environmental Control provisions of health product process, e.g., HVAC ✔
8. Used in critical supply of utilities in health products manufacturing, e.g., CIP/SIP, Purified Water ✔
9. Used in Laboratory to inspect and test health products ✔
10. Used in data records (word processing, spreadsheets, databases) to ensure integrity ✔
11. Wireless technology or interface employed for any of the above functions ✔
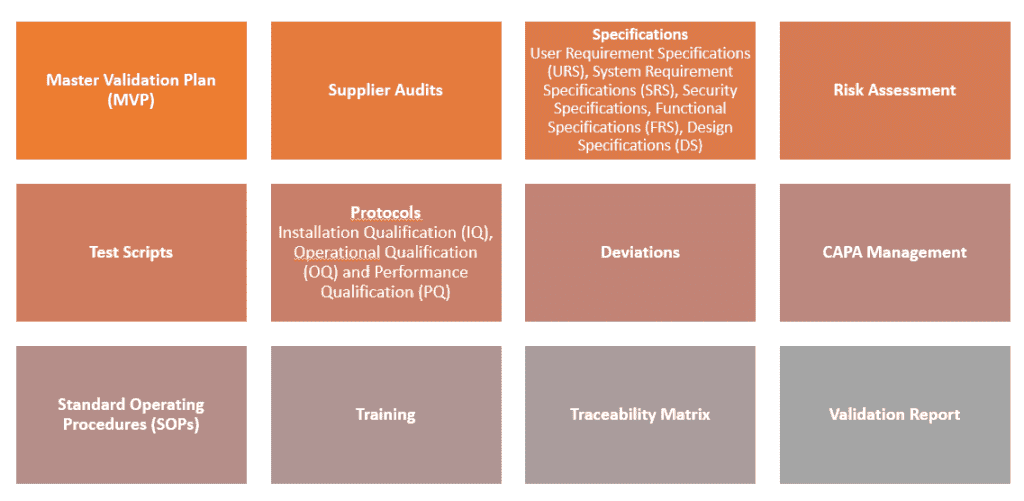
Deliverables
Documentation deliverables during the IQ, OQ and PQ phases of the computer system validation / software validation, include but are not limited to the following:
Contact us today for all your validation needs.
We are ready to support your Computer System Validation and medical device software validation needs.
